PhD Top Stories at UniTS: Matteo Bisetto (PhD in Nanotechnology)
Well-defined Cu2O photocatalysts for solar fuels and chemicals
Sourav Rej, Matteo Bisetto, Alberto Naldoni, Paolo Fornasiero
Journal of Materials Chemistry A9, 5915-5951 (2021)
DOI: 10.1039/D0TA10181H
In the last decades fossil fuels have been widely used for daily activities, contributing to a rapid increase of the Carbon Monoxide (CO2) concentration and the average temperatures. Scientific research focuses the attention on the development of new procedures to obtain “green” fuels and directly produce alcohols or hydrocarbons from recycled CO2. Due to the high stability of this molecule, an external energy is required to split CO2 and to obtain different products, among which the so-called Photocatalyst play a pivotal role.
Generally, these photocatalysts are inorganic metal oxides, and, because of their particular structure, they allow capture of a broad part of visible light to re-use it in the catalytic process. Furthermore, metal oxides are highly abundant and they can be easily modified in order to increase their overall performances. Among different photocatalysts, cuprous oxide (Cu2O) is a promising material for a wide range of processes thanks to its good photocatalytic behavior, its low cost and environmental impact and the possibility of tune its properties by changing the shape and size of the material. Crystal facet engineering allows to directly modify properties such as selectivity, absorption of radiation with different wavelength and reactivity toward a particular process.
In this work, we aim to discuss the different synthetic procedures for the preparation of diverse Cu2O structures with a particular shape and the role of this material in the field of photocatalysis. In particular, with our method it is possible to synthesize Cu2O nanocrystals with systematic shape evolution, from the stable cubic to other more complex structures, by changing the amount of added hydroxylamine hydrochloride (NH2OH·HCl), the reducing agent used during this synthesis. The obtained Scanning Electron Microscopy (SEM) images confirm the direct relationship between the final shape of Cu2O nanoparticles and the added amount of NH2OH·HCl (Figure 1).
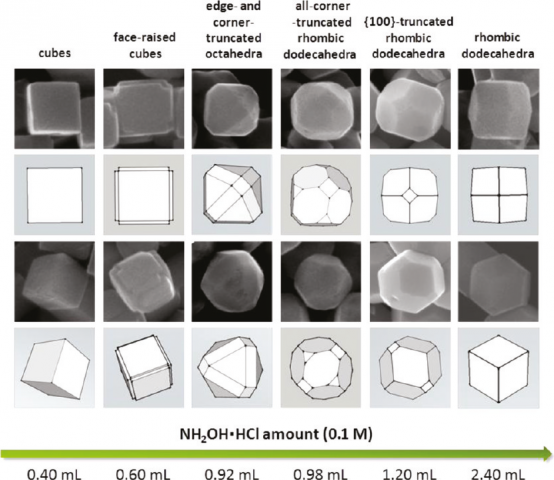
Figure 1: SEM images showing the shape evolution of Cu2O nanoparticles by increasing of NH2OH·HCl concentration during the synthesis.
Continue reading on PhD Top Stories 2021 at UniTS.
CONTACT
Matteo Bisetto, email: matteo.bisetto@phd.units.it.
Last updated on: 10/08/2021 - 08:49


